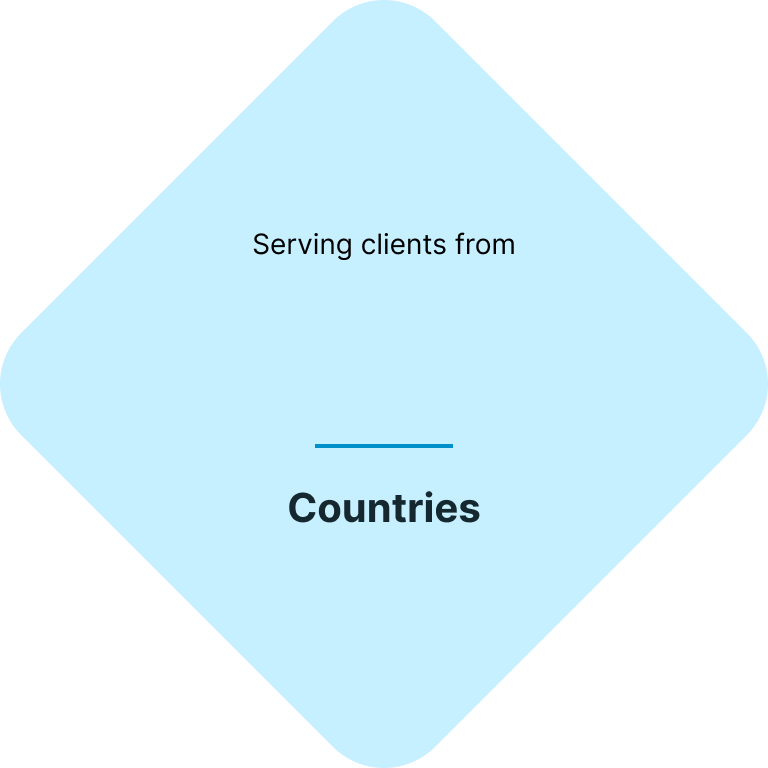
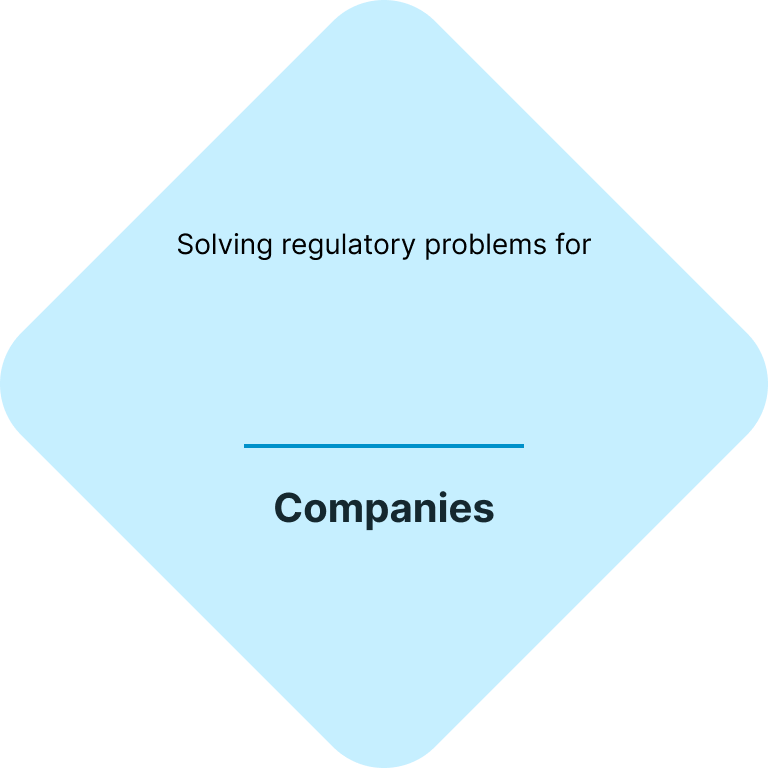
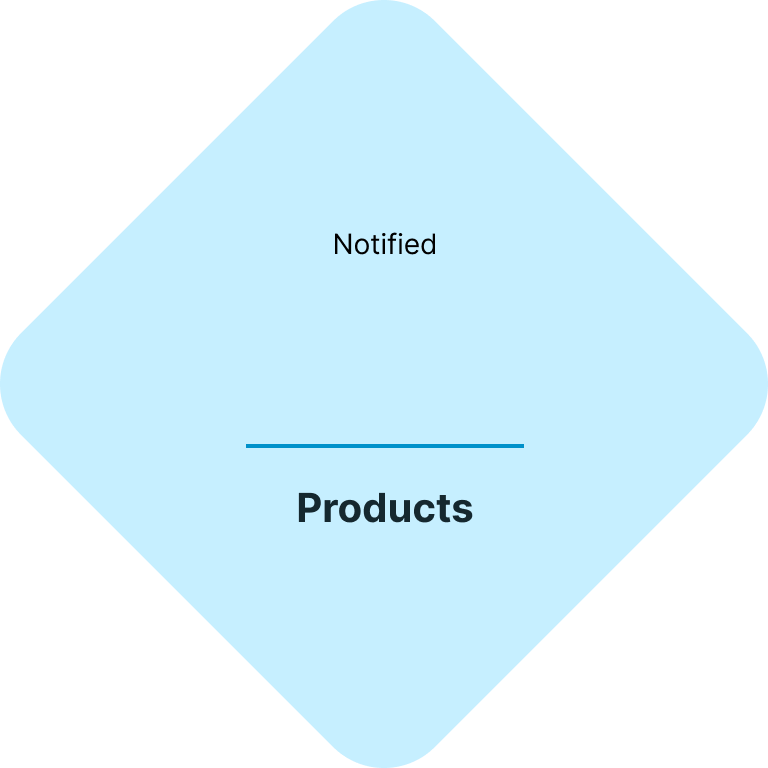
Your fast & easy cosmetics regulatory and testing solution
At CE.way, we help you bring your cosmetic products to the market quickly and efficiently. With a combination of our knowhow and in-house lab facility, we can offer all of the necessary regulatory and testing services, and seamlessly navigate you through the registration process of your cosmetic products.